Stereogenic carbon (and other tetrahedral stereogenic atoms!) are assigned a configuration designator based on a priority assignment among the four groups, and a standard method for describing the topologic arrangement of those priority groups. The methodology is straightforward; in practice there are a couple important things to stay aware of.
- Look at the four atoms connected to the stereocenter. Assign priority (1-4, high-to-low) based on:
- First, the atomic number of the atom;
- If no difference, the atomic mass of the atom;
- If no difference, then look at the priorities of the next-attached atoms for otherwise identical groups. Assign priority based on the first difference identified. Keep moving outward until a difference is found--if the two groups are identical the original carbon atom is not a stereocenter!
- The hard part: orient the molecule (either a model, a drawing, or a mental image) so that you view from the stereocenter to the 4th (low) priority group.
- Identify the order of rotation from Priority 1 to 2 to 3.
- Clockwise: the stereocenter is R.
- Counterclockwise: the stereocenter is S.
| Molecule |
Priority assignment |
|
R_BrClFMe.pdb |
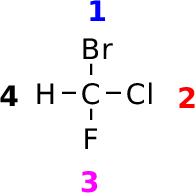 |
|
S_MeBnAmine.pdb |
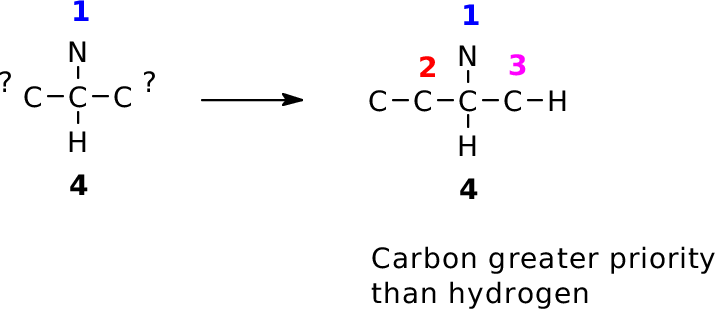 |
|
IP_CHexene.pdb |
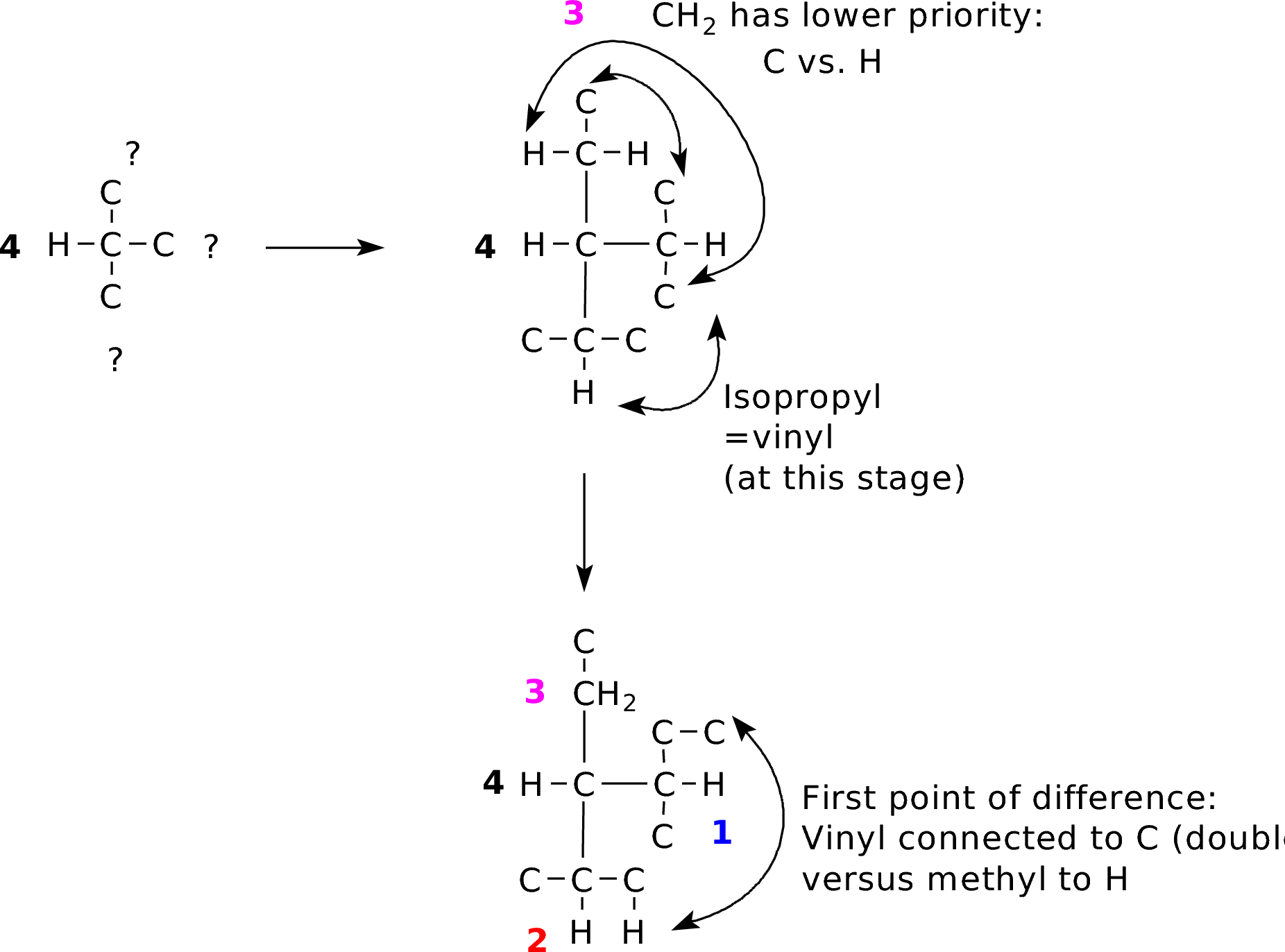 |
|
IP_CHexenol.pdb (Yes, this does have a second stereocenter. Try identifying its configuration.) |
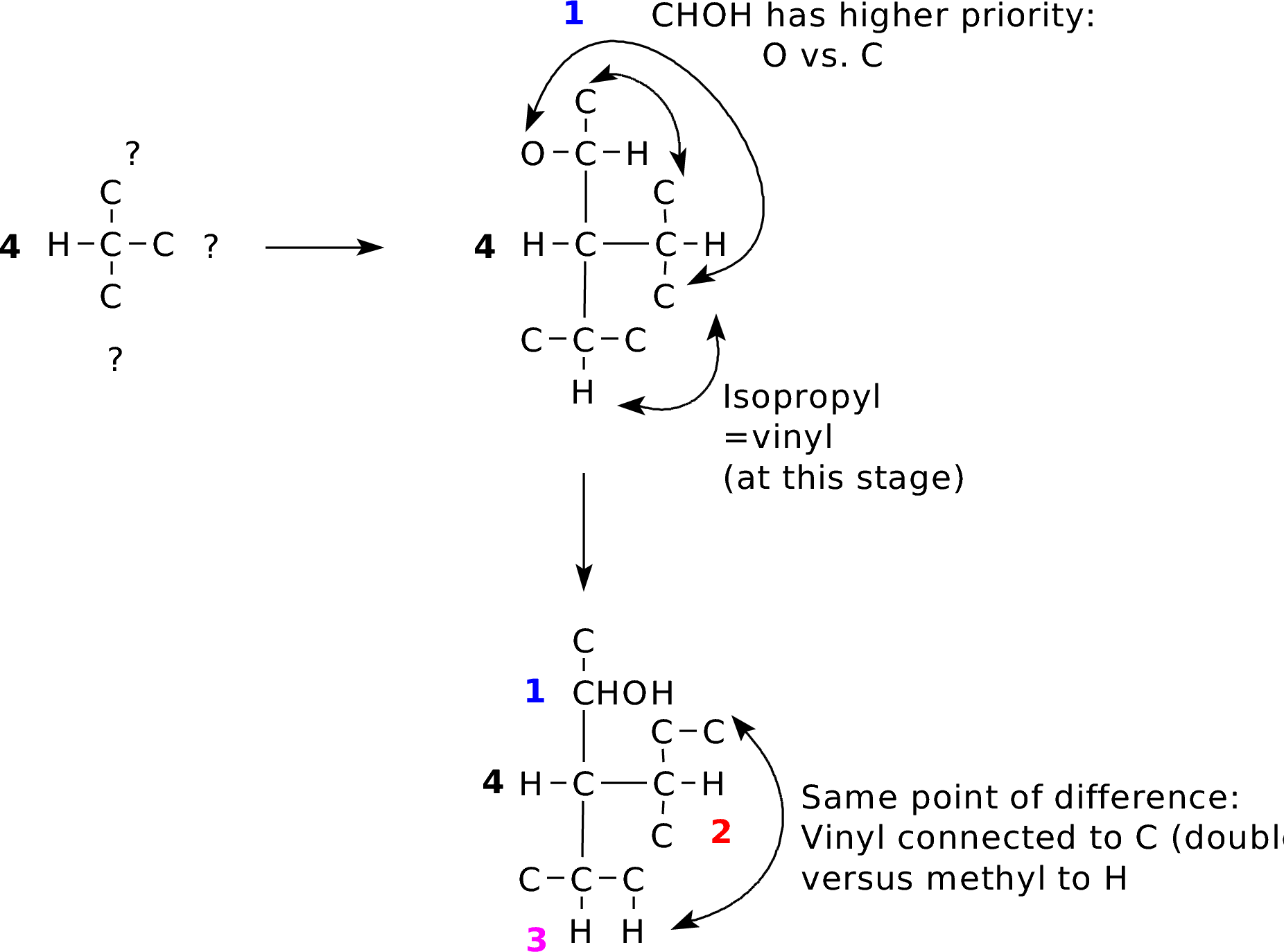 Double bonds are treated as two single bonds to the same element. This can get complicated, but always trace out connectivity to find the first point of difference. |
Page controls: Black background White background Spacefilling model Wireframe Ball & Stick Show Cahn-Prelog-Ingold Assignments |