Chemical Shift
The energy absorbed by nuclei when they undergo a spin flip is intrinsically measured against the irradiation frequency in Hz (usually expressed as MHz). As such it is instrument-specific. However, we find that the proportional frequency change, (Δν/ν0) is instrument independent and usually is on the order of a number of Hz versus an operating frequency in MHz. This ratio is expressed as a shift from a standard compound's frequency in parts per million (δ, ppm). Positive numbers are "downfield" (requiring a lower magnetic field to achieve the spin flip) or "deshielded" (less electron density and less magnetic shielding from the instrument's magnetic field B0).
The reference point (0 ppm) is the chemical shift of tetramethylsilane, (CH3)4Si.
Here is a table of typical 1H chemical shifts:
| Chemical Environment of
the Hydrogen |
10+ |
9 |
8 |
7 |
6 |
5 |
4 |
3 |
2 |
1 |
0 |
| Alkane CH-CR3 |
0.8-1.9 |
||||||||||
| Allylic, Benzylic, ketone =C-CH, Ph-CH, CH-C=O |
1.8-2.5 |
||||||||||
| Alkyne C=C-H |
2.5-3.5 |
||||||||||
| Alkyl halide CH-X |
3-5 |
||||||||||
| Ether/alcohol/ester CH-O |
3.5-5.5 |
||||||||||
| Alkene =C-H |
4.5-7.0 |
||||||||||
| Aromatic Ph-H |
7-9 but some 6.5-9.5 |
||||||||||
| Aldehyde proton RC(=O)-H |
9-10 |
||||||||||
| Carboxylic Acid RCO2H |
9.5
and above (broad) |
||||||||||
| 10+ |
9 |
8 |
7 |
6 |
5 |
4 |
3 |
2 |
1 |
0 |
|
Some examples
SpectrumPlease note that many of these spectra are simulations based on data in the Spectral Database for Organic Compounds maintained by the Japanese AIST. Others are processed from raw data maintained by Pacific Lutheran University. We thank both organizations for access to this data. |
Structure & Notes |
methoxyacetonitrile.jdx |
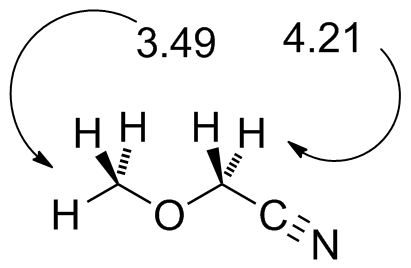 Note the effect of the cyano group in further deshielding the CH2 protons. |
|
MTBE.jdx |
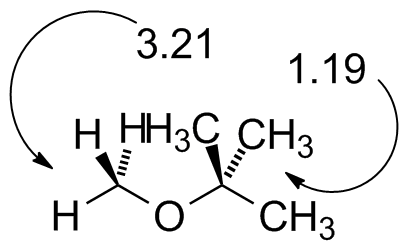 Compare to the diagram on Page 387 at the beginning of the chapter. (No TMS in this spectrum) |
|
22Dimethylpropanol.jdx |
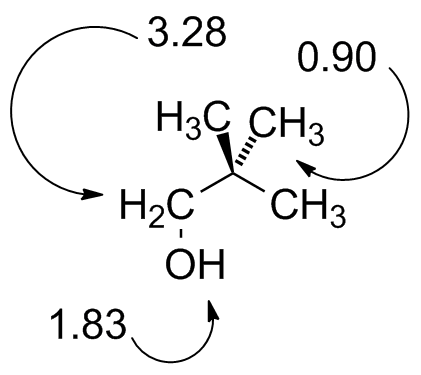 |
|
BnOH.jdx |
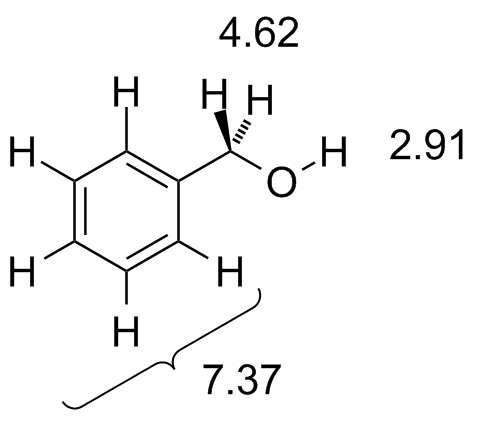 The aromatic protons all come at about the same chemical shift and we see a single transition there. Note as well the CH2 protons are a little further downfield--the additive effect of the benzylic position plus the oxygen. |
|
Cyclohexene.jdx |
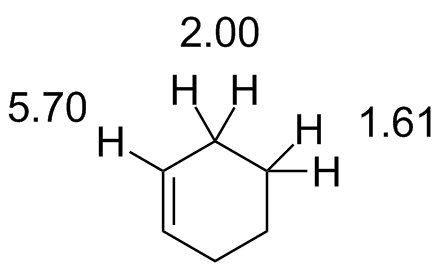 |