Analysis of Calorimetry Data
This should be very similar to the analysis of data for calculating the calorimeter
constant. There are several new twists, but they should be obvious if you carefully
think about what you are doing.
Recall:
In general, the relation between what you measure (change in temperature of the thermostat
water) and the chemical change (the heat generated by combustion) is given by the simple
equation:
qobs = CΔT
Here
- qobs is the energy released by combustion in calories;
- C is the calorimeter constant in calories per °C;
- ΔT is the temperature change induced by the heat absorption,
in °C.
(We could certainly use SI units of Joules instead of calories, though organic
thermochemistry has traditionally used the latter units.)
Measuring ΔT is somewhat involved. The calorimeter is
continually losing heat to the environment, and heat transfer from the inside of the bomb
to the water is not instantaneous. Therefore, we have to extrapolate the cooling
curve before ignition (the five minutes of data collection at first) and the cooling curve
after ignition to a "t0" point. This won't exactly be the
ignition time; it is the time at which the average temperature is passed. Take a
look at the following data set.
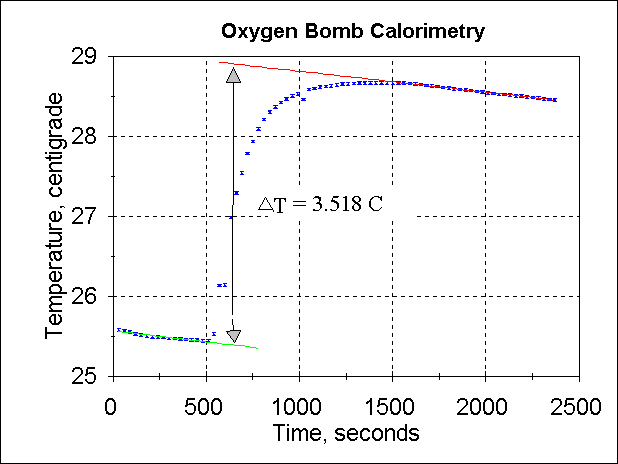
First, find the most linear portion of the two cooling curves. You will have to be
judicious in your choice! For example, if you do not use points late enough on the
upper cooling curve, Thigh will be too low and you will get inaccurate
results. Use a spreadsheet to perform linear regression for both cooling curves.
If r2 is not greater than 0.97, you need
to restrict the number of points used.
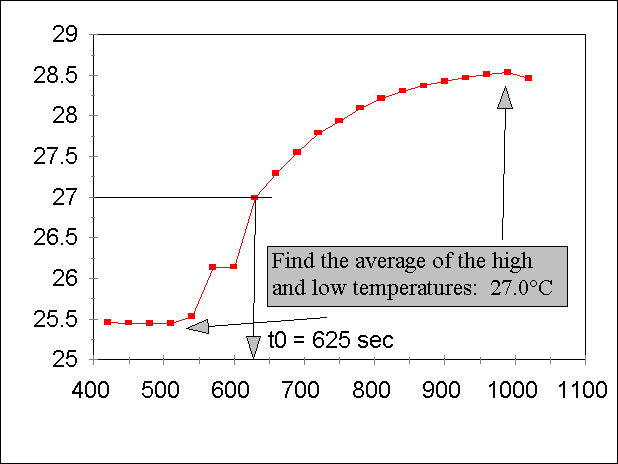
Next, you have to find the "t0"
point. Find the high and low temperature that you actually measure, and take
the average. Next, expand the X-axis for the T vs. time data, and find the time at
which the temperature passes this Tavg point. You will probably have to
interpolate your data; see below. Remember, you would like as many significant
figures as possible!
Use the slope and intercept of each cooling curve to find Tlow
and Thigh for X = t0. The difference between these two
temperatures is ΔT, the theoretical temperature that
would be seen if heat transfer were instantaneous. This is the value you use in the
equation above.
Now we come to some differences with the calculation of C. You know C, of course,
so ΔT now tells you (by appropriate algebra) the heat released
by combustion of your ester.
However, qobs is now a composite of ΔEc
for the ester, and ΔEc for the iron wire. From
qobs, you must subtract ΔEiron;
if you have any soot, this represents heat that should be added. So,
ΔEc(ester) = qobs
- ΔEiron + ΔEsoot
The energy released on combustion also counts PV work done by the system; this must be
properly accounted for to arrive at the desired enthalpy of combustion:
ΔHc = ΔEc
+ RTΔngas
Calculate Δngas based on the stoichiometry
of the combustion reaction. R is the ideal gas constant: 1.987 cal/(mol-K) or
8.314 J/(mol-K).
Forward to Gaussian page
Back to Calorimetry page
Back to CH362 Home Page
Comments to: K. Gable

