Drawing Conventions
One of the major challenges for someone learning organic chemistry is to make the mental connection between a 2-dimensional drawing of a molecule, and the real molecule in 3 dimensions. There are several standard conventions that help us communicate the spatial properties of a molecule.
Wedge-and-Hash
The first is the wedge-and-hash convention.

The solid wedge implies the group at the wide end is above the plane; for the hash, the group at the wide end is behind the plane. Thus, 1-phenylethanol can be depicted as:
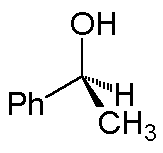
(A 3-dimensional ball-and-stick model is on the right.)
Heavy/dashed bond lines
A somewhat related convention for acyclic compounds uses bold bonds in place of a wedge,
and a dashed line in place of the hash marks. The carbon chain is depicted as a
zig-zag chain of C-C bonds (remember that a terminal line with no group designator implies
a methyl group):
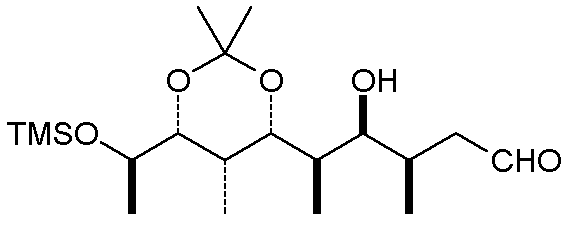
One thing to note is that the actual conformation of the molecule may be different than
the simplest depiction. Rotations about single bonds can often relieve steric
congestion, and internal attractive interactions (H-bonds and Van der Waals attractions)
will usually cause a linear chain to curl up somewhat. The molecule above, when
minimized at the MM+ level, looks like this:
The Stereochemical Dot
In complex cyclic structures, it may be difficult to completely depict the locations of
all hydrogens. A convention developed in steroid chemistry is the
"stereochemical dot;" this explicitly depicts a b-hydrogen
(one lying above the plane of the carbon). Two alternative depictions of cholesterol
are shown below.
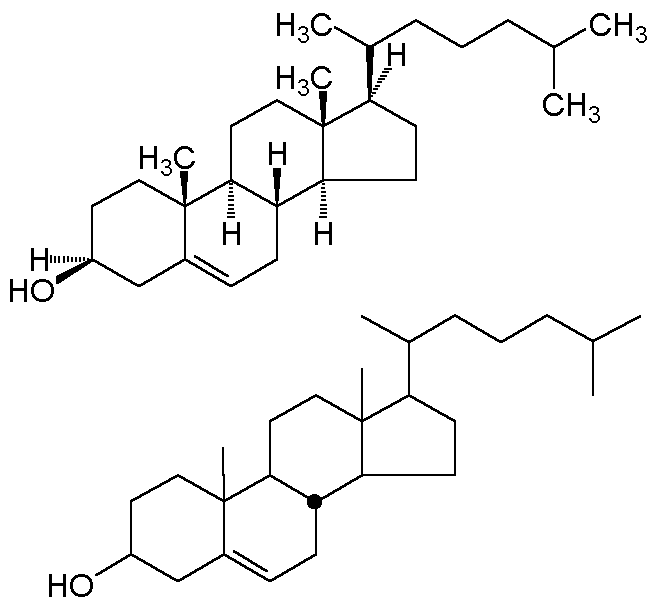
In the bottom convention, and exocyclic bond is assumed to be "up" unless
specifically given a dashed line. Ring junctions must be explicitly designated; the
one stereochemical dot makes the B-C and C-D ring junctions trans.
The Diamond Lattice
See this separate page.
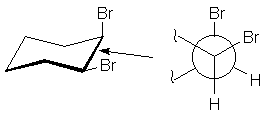
Newman Projections
Often, the arrangement of groups about one bond reveal important aspects of molecular
behavior. A useful method of examining such interactions was devised by Dr. Melvin
Newman. You imagine yourself looking down the bond, and represent the bond by a
circle. The atom in front has bonds radiating from the center of the circle.
The atom in back has bonds radiating from the circle. Thus, the 1,2-bond of cis-dibromocyclohexane
may be represented as follows:
Fischer Projections
Fischer projections are most commonly seen in carbohydrate and amino acid chemistry.
The historical source of this convention stems from the problem of assigning absolute
stereochemistry; prior to the advent of X-ray diffraction techniques, it was possible to
deduce relative stereochemistry in molecules with two or more stereocenters, but not
absolute stereochemistry. Professor Emil Fischer invented a convention that allowed
depiction of relative stereochemistry, but was ambiguous about absolute
stereochemistry. A crossing of vertical and horizontal lines to represent
tetrahedral carbon could have either of two meanings; once diffraction analysis gave us
absolute stereochemical information, we recognize the convention as indicating the
vertical bonds going behind the plane, and the horizontal bonds coming out of the plane:
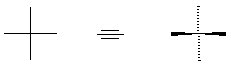
Thus, the open form of D-glucose has the following representations (Fischer projection on the left, wedge-and-hash convention on the right):
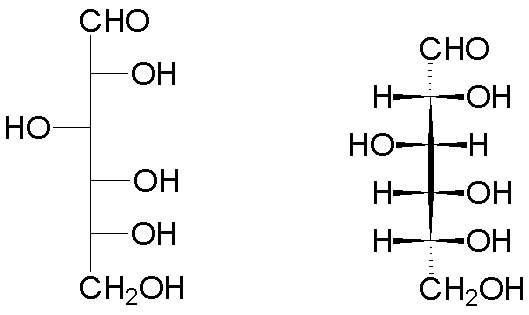
Back to CH630 Home Page
Last updated: 8/5/97
Comments to K. Gable