- Steric bulk of the base (tert-butoxide gives more than ethoxide). However, remember that smaller bases are also better nucleophiles and will do more substitution.
- Branching in the substrate. This may create steric hindrance for the base approaching the hydrogen that leads to a Zaitsev product. Slow down that reaction, and the production of the Hofmann product becomes a larger part of the outcome.
- Poor leaving groups. This pushes us to a transition state with less C-X cleavage, less double bond character, and less of the thermodynamic influence from substitution of the incipient alkene.
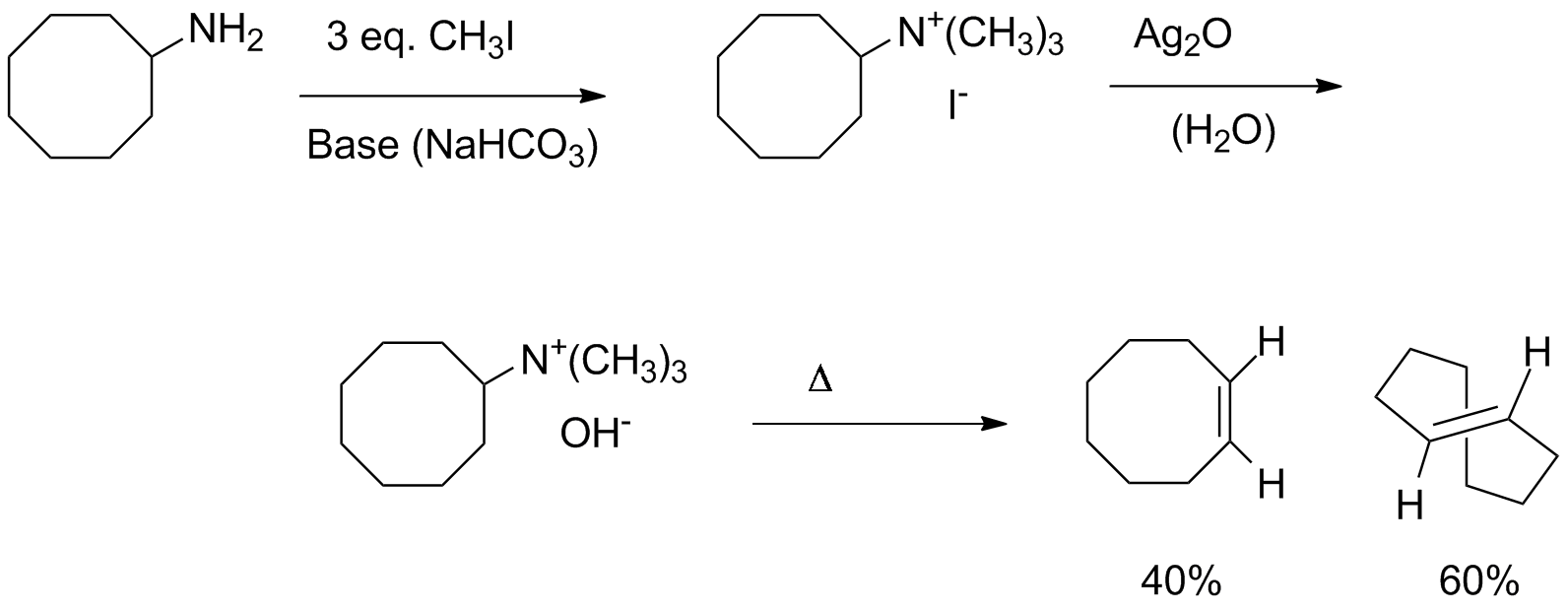
An excess of iodomethane does an SN2 reaction repeatedly on the nitrogen of an amine, with base neutralizing the HI byproduct. Then silver oxide precipitates AgI leaving hydroxide as the counterion. This basic anion associates with the positively charged nitrogen, leading to syn elimination (predominantly).