| |
CH4/535Structure Determination by Spectroscopic MethodsMosher ester derivatives for assignment of absolute stereochemistry
|
 |
| |
||
| |
||
| |
||
| |
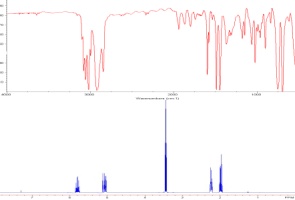
Assignment of absolute stereochemistry is usually impossible by NMR, since the enantiomers of any stereogenic molecule display the identical spectra. However, creating a diastereomeric relationship breaks this symmetry, and in the case of alcohols, a pair of diastereomers can be made by making the (methoxy)(phenyl)(trifluoromethyl)acetate esters--one from each enantiomer. The resultant esters will show the impact of the diamagnetic ring current from the phenyl substituent. The methoxy and trifluoromethyl groups tend to orient the phenyl in such a manner as to cover one of the two substituents on the (secondary) alcohol in a regular fashion. (Diagram adapted from Riguera, et al., Tetrahedron: Asymm., 2000, 11, 2781-2791. Note that on-campus internet access or use of VPN or a campus proxy server will be needed to access this article) The S ester (made from the R chloride) shifts the front group upfield, and the R ester (made from the S chloride) shifts the back group upfield. We'll define ΔδSR as the difference in chemical shift at each position between the ester made from the R acid chloride and that for the same position from the ester made from the S acid chloride. If the front group is L1 and the back is L2, then the L1 group will show ΔδSR that is positive, the L2 group will show ΔδSR that is negative.  In this example, the two esters are made from the acid chloride. (Note that R and S change because the ester has different priorities than the acid chloride: the method depends on the defined fonfiguration of the reagent.)  In this case the L1 group is the acetal ring, and the L2 group is the chain with the pivalate. |