| |
CH4/535Structure Determination by Spectroscopic Methods (CRN 38538, 38539)A substitution problem
|
 |
| |
||
| |
||
| |
||
| |
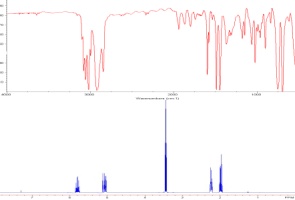
A student performed the following reaction, and in preparation for subsequent transformations, it was critical to know with certainty which of two hydroxyls was methylated:  Only one product was isolated; MS confirmed monomethylation, and the IR confirmed presence of an OH and retention of the coumarin core. It was therefore necessary to attempt as complete an assignment as possible. 1H NMR: 13C NMR: The COSY confirms coupling of the two aromatic and two vinylic protons, so it is of little direct use. Calculation of the 13C shifts shows very little difference other than to broadly confirm some assignments: |
13C calculated/actual shifts:|||
| Carbon number: |

|
Actual: | |
| 1 | 135.6 | 136.8 | 132.7 |
| 2 | 117.7 | 124.0 | 113.5 |
| 3 | 120.3 | 120.2 | 119.0 |
| 4 | 113.4 | 112.2 | 107.0 |
| 5 | 142.5 | 150.8 | 150.0 |
| 6 | 144.0 | 135.7 | 142.0 |
|
HSQC: | |||